WebPeptide Bond. Here, well take a closer look at ribosomes and tRNAs. In eukaryotic translation, there are also ribosomal subunits which must come together around an mRNA, but the process is a whole lot more complex with lots of protein-RNA interactions and protein-protein interactions. What are the functions of the three major types of RNA molecules involved in protein synthesis? In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other.One loses a hydrogen and oxygen from its carboxyl group (COOH) and One end of the tRNA binds to a specific amino acid (amino acid attachment site) and the other end has an anticodon that will bind to an mRNA codon. [23] described the specific cleavage by leishmanolysin from L. mexicana of a synthetic peptide with Val in the P1 site, Thr in the P1 site, and lysines in both the P2 and P3 sites (Figure 277.1). Whereas most peptide bonds exist in the trans configuration to keep the side chains (R-groups) as far apart as possible, the peptide bond that involves the NH group of the rigid pyrrolidone ring of proline can occur in both trans and cis arrangements (Figure 4.3). How does the structure of RNA differ from the structure of DNA? It is coded by DNA, then it's transcribed by special polymerase, spliced and there we have it. The frequent presence of tyrosine at the P1 site and of basic amino acids at the P2 and P3 sites of susceptible peptides suggests that some specificity exists at these subsites [22]. WebPeptide bonds form between the adjacent amino acids to form the polypeptide (protein). It introduces a basic functional group (i.e., amino group) that can be protonated and increase the hydrophilicity.131 It has been widely used in the design of metabolically stable analogs of natural peptides.132 For example, an amide bond surrogate [CH2NH] between Tyr1 and Gly2 of Met-enkephalin improved the potency by twofold compared with the unmodified peptide, in combination with a methioninol replacing Met at the C-terminus.133 On the other hand, replacement of the same amide bond with a hydrocarbon analog (CH2CH2) led to a significant decrease in activity compared with its reduced bonded analog. Dysregulated proteolytic activity could be responsible for the disruption in the homeostatic balance of a biological system and can result in any number of poor biological outcomes. (a) DNA is typically double stranded, whereas RNA is typically single stranded. Future plans, financial benefits and timing can be huge factors in approach.  [11] This non-enzymatic process is thus not accelerated by transition state stabilization, but rather by ground-state destabilization. John W. Pelley PhD, in Elsevier's Integrated Biochemistry, 2007.
[11] This non-enzymatic process is thus not accelerated by transition state stabilization, but rather by ground-state destabilization. John W. Pelley PhD, in Elsevier's Integrated Biochemistry, 2007. 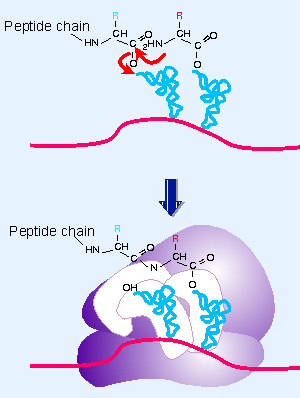 Learn more about how this process works in the next article, on the, Posted 7 years ago.
Learn more about how this process works in the next article, on the, Posted 7 years ago.  It is the base pairing between the tRNA and mRNA that allows for the correct amino acid to be inserted in the polypeptide The Structural Basis of Ribosome Activity in Peptide Bond Synthesis. Science 289 no. This bond links two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another. A peptide bonds forms between the two amino acids. Time course experiments were performed using 250nM of each peptide and 8nM of leishmanolysin from L. major at 37C in Tris-buffer saline at pH7.5. The active site of each aminoacyl-tRNA synthetase fits an associated tRNA and a particular amino acid like a "lock and key." How does the right amino acid get linked to the right tRNA (making sure that codons are read correctly)? The fluorogenic internally quenched dansyl heptapeptide [24] derived from these studies presented a smaller Kcat of 14.1s1 compared to that for the cytochrome c peptide 94102 (Kcat=40s1), but its substantially greater Km (1.6106M) resulted in a kcat/Km ratio of 8.8106M1s1. The two joined amino acids are called a dipeptide. consent of Rice University. If a cell requires a certain protein to be synthesized, the gene for this product is turned on and the mRNA is synthesized through the process of transcription (see RNA Transcription). Therefore if a protein were to contain only one strand of amino acids it could be called a peptide as you have noticed. 20 At one end, the tRNA has an anticodon of 3'-UAC-5', and it binds to a codon in an mRNA that has a sequence of 5'-AUG-3' through complementary base pairing. Rotaviruses, which cause severe gastroenteritis in children and other immunocompromised individuals, are examples of double-stranded RNA viruses. WebThese proteins are also called polypeptides. Bailey, in Methods in Enzymology, 2009.
It is the base pairing between the tRNA and mRNA that allows for the correct amino acid to be inserted in the polypeptide The Structural Basis of Ribosome Activity in Peptide Bond Synthesis. Science 289 no. This bond links two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another. A peptide bonds forms between the two amino acids. Time course experiments were performed using 250nM of each peptide and 8nM of leishmanolysin from L. major at 37C in Tris-buffer saline at pH7.5. The active site of each aminoacyl-tRNA synthetase fits an associated tRNA and a particular amino acid like a "lock and key." How does the right amino acid get linked to the right tRNA (making sure that codons are read correctly)? The fluorogenic internally quenched dansyl heptapeptide [24] derived from these studies presented a smaller Kcat of 14.1s1 compared to that for the cytochrome c peptide 94102 (Kcat=40s1), but its substantially greater Km (1.6106M) resulted in a kcat/Km ratio of 8.8106M1s1. The two joined amino acids are called a dipeptide. consent of Rice University. If a cell requires a certain protein to be synthesized, the gene for this product is turned on and the mRNA is synthesized through the process of transcription (see RNA Transcription). Therefore if a protein were to contain only one strand of amino acids it could be called a peptide as you have noticed. 20 At one end, the tRNA has an anticodon of 3'-UAC-5', and it binds to a codon in an mRNA that has a sequence of 5'-AUG-3' through complementary base pairing. Rotaviruses, which cause severe gastroenteritis in children and other immunocompromised individuals, are examples of double-stranded RNA viruses. WebThese proteins are also called polypeptides. Bailey, in Methods in Enzymology, 2009. 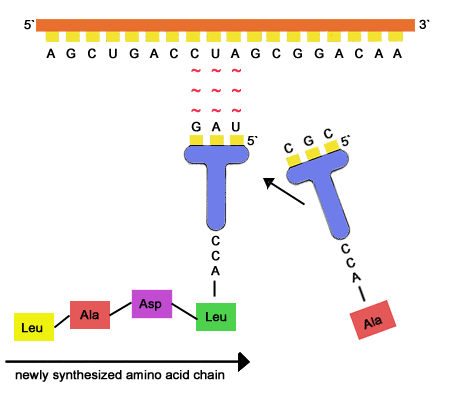 L/F-transferase is the sole member of this class of enzyme found in The partial double bond renders the amide group planar, occurring in either the cis or trans isomers. ; But since most proteins are not only composed of one chain you cannot call them a peptide, but a polypeptide. WebProtein synthesis The mRNA binds to the small subunit of the ribosome tRNA carrying methionine (amino acid) binds at the start codon AUG. A peptide bonds forms between the two amino acids. As we saw briefly in the introduction, molecules called transfer RNAs (tRNAs) bring amino acids to the ribosome. Ribosomes are composed of rRNA and protein. The ribosome moves forward on the mRNA, codon by codon, as it is read and translated into a polypeptide (protein chain). citation tool such as, Authors: Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister, Brian M. Forster. (b) RNA contains the pyrimidine uracil in place of thymine found in DNA. The degradome has been shown to affect many crucial biochemical pathways. Menu. The study of proteolysis goes back to the first half of the nineteenth century with the description of pepsin by Schwann in 1836 and, successively, of trypsin by Corvisart in 1856. Model of the small and large subunits of the ribosome. (complementary base pairing) A second tRNA anticodon binds and pairs with the next codon. This process WebA peptide bond is basically an amide-type of covalent chemical bond. A. Direct link to tyersome's post Another good question. This process Conformational protein folding is usually much faster (typically 10100ms) than cis-trans isomerization (10100s). Even though RNA is single stranded, most types of RNA molecules show extensive intramolecular base pairing between complementary sequences within the RNA strand, creating a predictable three-dimensional structure essential for their function (Figure 10.20 and Figure 10.21).
L/F-transferase is the sole member of this class of enzyme found in The partial double bond renders the amide group planar, occurring in either the cis or trans isomers. ; But since most proteins are not only composed of one chain you cannot call them a peptide, but a polypeptide. WebProtein synthesis The mRNA binds to the small subunit of the ribosome tRNA carrying methionine (amino acid) binds at the start codon AUG. A peptide bonds forms between the two amino acids. As we saw briefly in the introduction, molecules called transfer RNAs (tRNAs) bring amino acids to the ribosome. Ribosomes are composed of rRNA and protein. The ribosome moves forward on the mRNA, codon by codon, as it is read and translated into a polypeptide (protein chain). citation tool such as, Authors: Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister, Brian M. Forster. (b) RNA contains the pyrimidine uracil in place of thymine found in DNA. The degradome has been shown to affect many crucial biochemical pathways. Menu. The study of proteolysis goes back to the first half of the nineteenth century with the description of pepsin by Schwann in 1836 and, successively, of trypsin by Corvisart in 1856. Model of the small and large subunits of the ribosome. (complementary base pairing) A second tRNA anticodon binds and pairs with the next codon. This process WebA peptide bond is basically an amide-type of covalent chemical bond. A. Direct link to tyersome's post Another good question. This process Conformational protein folding is usually much faster (typically 10100ms) than cis-trans isomerization (10100s). Even though RNA is single stranded, most types of RNA molecules show extensive intramolecular base pairing between complementary sequences within the RNA strand, creating a predictable three-dimensional structure essential for their function (Figure 10.20 and Figure 10.21). For example, the tRNA for phenylalanine has an anticodon of 3'-AA, The rules of wobble pairing ensure that a tRNA does not bind to the wrong codon. Due to interference from solvents and components of biological buffers, absorbance at 214 and 220nm is often used as an alternative to measure proteins and peptides. Show transcribed image text. The ribosome's peptidyl transferase catalyses the transfer of the growing polypeptide chain from the P site tRNA to the amino group of the A site amino acid. The chelating peptide, Z-Tyr-Leu-hydroxamate inhibits leishmanolysin in the high micromolar range (17M), which is much lower than the millimolar concentrations needed for inhibition with 1,10-phenanthroline [22]. (complementary base pairing) A second tRNA anticodon binds and pairs with the next codon. We use cookies to help provide and enhance our service and tailor content and ads. The ribosome moves along the mRNA. Atypical base pairsbetween nucleotides other than A-U and G-Ccan form at the third position of the codon, a phenomenon known as, Wobble pairing doesn't follow normal rules, but it does have its own rules. WebA ribosome has three binding sites, each of which has a distinct function in the tRNA-mRNA interactions. WebWhereas most peptide bonds exist in the trans configuration to keep the side chains (R-groups) as far apart as possible, the peptide bond that involves the NH group of the rigid pyrrolidone ring of proline can occur in both trans and cis arrangements (Figure 4.3).However, X-ray data suggest that the trans form occurs more frequently in proteins WebWhen two amino acids form a dipeptide through a peptide bond, it is a type of condensation reaction. You found me for a reason. So, a tRNA is is L shaped in 3D and clover leaf shaped in 2D? Be sure of your position before leasing your property. refold into a different and infectious three-dimensional shape that kills cells in the brain and nervous system. Direct link to Ivana - Science trainee's post when tRNA has bound amino. More specific nomenclature can indicate the number of amino acids in the polypeptide, e.g., dipeptide (two amino acids) or oligopeptide (relatively few amino acids). The numbers indicate the specific activity of leishmanolysin on each peptide in moles of peptide bond cleaved per second per mole of leishmanolysin [22]. The ribosome will then move along the mRNA template by one codon. The trans form is preferred overwhelmingly in most peptide bonds (roughly 1000:1 ratio in trans:cis populations). Some tRNAs can form base pairs with more than one codon. This creates a peptide bond between the C terminus of the growing polypeptide chain and the A site amino acid. are licensed under a, Unique Characteristics of Prokaryotic Cells, Unique Characteristics of Eukaryotic Cells, Prokaryote Habitats, Relationships, and Microbiomes, Nonproteobacteria Gram-Negative Bacteria and Phototrophic Bacteria, Isolation, Culture, and Identification of Viruses, Using Biochemistry to Identify Microorganisms, Other Environmental Conditions that Affect Growth, Using Microbiology to Discover the Secrets of Life, Structure and Function of Cellular Genomes, How Asexual Prokaryotes Achieve Genetic Diversity, Modern Applications of Microbial Genetics, Microbes and the Tools of Genetic Engineering, Visualizing and Characterizing DNA, RNA, and Protein, Whole Genome Methods and Pharmaceutical Applications of Genetic Engineering, Using Physical Methods to Control Microorganisms, Using Chemicals to Control Microorganisms, Testing the Effectiveness of Antiseptics and Disinfectants, History of Chemotherapy and Antimicrobial Discovery, Fundamentals of Antimicrobial Chemotherapy, Testing the Effectiveness of Antimicrobials, Current Strategies for Antimicrobial Discovery, Virulence Factors of Bacterial and Viral Pathogens, Virulence Factors of Eukaryotic Pathogens, Major Histocompatibility Complexes and Antigen-Presenting Cells, Laboratory Analysis of the Immune Response, Polyclonal and Monoclonal Antibody Production, Anatomy and Normal Microbiota of the Skin and Eyes, Bacterial Infections of the Skin and Eyes, Protozoan and Helminthic Infections of the Skin and Eyes, Anatomy and Normal Microbiota of the Respiratory Tract, Bacterial Infections of the Respiratory Tract, Viral Infections of the Respiratory Tract, Anatomy and Normal Microbiota of the Urogenital Tract, Bacterial Infections of the Urinary System, Bacterial Infections of the Reproductive System, Viral Infections of the Reproductive System, Fungal Infections of the Reproductive System, Protozoan Infections of the Urogenital System, Anatomy and Normal Microbiota of the Digestive System, Microbial Diseases of the Mouth and Oral Cavity, Bacterial Infections of the Gastrointestinal Tract, Viral Infections of the Gastrointestinal Tract, Protozoan Infections of the Gastrointestinal Tract, Helminthic Infections of the Gastrointestinal Tract, Circulatory and Lymphatic System Infections, Anatomy of the Circulatory and Lymphatic Systems, Bacterial Infections of the Circulatory and Lymphatic Systems, Viral Infections of the Circulatory and Lymphatic Systems, Parasitic Infections of the Circulatory and Lymphatic Systems, Fungal and Parasitic Diseases of the Nervous System, Fundamentals of Physics and Chemistry Important to Microbiology, Taxonomy of Clinically Relevant Microorganisms. Geometry of a peptide (amide) linkage. In eukaryotes, synthesis, cutting, and assembly of rRNA into ribosomes takes place in the nucleolus region of the nucleus, but these activities occur in the cytoplasm of prokaryotes. The three main types of RNA directly involved in protein synthesis are messenger RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA). If DNA serves as the complete library of cellular information, mRNA serves as a photocopy of specific information needed at a particular point in time that serves as the instructions to make a protein. The partial double-bond character of the NC bond in the transition state probably best represents what exists in nature. WebA ribosome has three binding sites, each of which has a distinct function in the tRNA-mRNA interactions. You are correct, this article deals with prokaryotic translation. WebAter each tRNA locksamino acids nto place, a peptide bond forms between the amin acid the tRNA is carrying and the amino acid aready there. binds to its complementary mRNA codon. The ribosome's peptidyl transferase catalyses the transfer of the growing polypeptide chain from the P site tRNA to the amino group of the A site amino acid. {\displaystyle \omega } A ribosome is shown with mRNA and tRNA. Both subunits are made up of both ribosomal RNA and proteins. Both of these mechanisms for lowering the activation energy have been observed in peptidyl prolyl isomerases (PPIases), which are naturally occurring enzymes that catalyze the cis-trans isomerization of X-Pro peptide bonds. Significant delocalisation of the lone pair of electrons on the nitrogen atom gives the group a partial double-bond character. The linear sequence of amino acids is read from left to right, with the amino terminal on the left. The bonds on either side of the -carbon (i.e., between the -carbon and the nitrogen, and between the -carbon and the carbonyl carbon) are strictly single bonds. The ribosomal catalytic centres that decode mRNA, trigger GTP hydrolysis and mediate the formation of peptide bonds between amino acids are distant from one another. Hi, where does the Amino Acid comes from? binds to its complementary mRNA codon. A new tRNA (in this case, one bearing Phe) will bind to the newly exposed codon in the A site, and the process can then repeat. a. cysteine-alanine b. proline-cysteine c. glycine-cysteine d. alanine-alanine e. threonine-glycine For the peptide bond, bond angles and bond lengths indicate that carbonnitrogen bonds have a significant amount of double-bond character, and that the C, O, N, and H atoms all lie in the same plane. The other end of the tRNA carries the amino acid methionine (Met), which is the the amino acid specified by the mRNA codon AUG. Language links are at the top of the page across from the title. are not subject to the Creative Commons license and may not be reproduced without the prior and express written This bond links two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another. Rich. The designation for the angle of rotation of the -carbonnitrogen bond is , whereas that of the -carboncarbonyl carbon bond is . Our mission is to improve educational access and learning for everyone. Are you sure youre using the best strategy to net more and decrease stress? = Thus, a lot of proteases play key roles in several biological processes, such as cell cycle progression, tissue remodeling, neuronal outgrowth, haemostasis, wound healing, angiogenesis, digestion, blood clotting, immunity, host defense, pathogenic infection, viral replication, disease progression, and apoptosis (Farady and Craik, 2010; Perez-Silva et al., 2016). To log in and use all the features of Khan Academy, please enable JavaScript in your browser. WebAfter the peptide bond is formed, the ribosome shifts, or translocates, again, thus causing the tRNA to occupy the E site. Different forms of these enzymes are found in the different kingdoms of life and have been identified to be central to a wide variety of cellular processes. Direct link to Emily's post Replication is making mor, Posted 4 years ago.
 This effect is due to amidoimido tautomerization. Peptide bonds have a planar trans configuration and undergo very little rotation or twisting around the amide bond that links the -amino nitrogen of one amino acid to the carbonyl carbon of the next amino acid (Figure 4.2). During translation, the two subunits come together around a mRNA molecule, forming a complete ribosome. 90 Many sales people will tell you what you want to hear and hope that you arent going to ask them to prove it. Does the Wobble Position apply to START and STOP codons as well? The polymerization of amino acids produces a linear molecule referred to generically as a polypeptide (Fig. and you must attribute OpenStax. This is the pathway followed in proteolysis and, more generally, in NO acyl exchange reactions such as those of inteins. [23].
This effect is due to amidoimido tautomerization. Peptide bonds have a planar trans configuration and undergo very little rotation or twisting around the amide bond that links the -amino nitrogen of one amino acid to the carbonyl carbon of the next amino acid (Figure 4.2). During translation, the two subunits come together around a mRNA molecule, forming a complete ribosome. 90 Many sales people will tell you what you want to hear and hope that you arent going to ask them to prove it. Does the Wobble Position apply to START and STOP codons as well? The polymerization of amino acids produces a linear molecule referred to generically as a polypeptide (Fig. and you must attribute OpenStax. This is the pathway followed in proteolysis and, more generally, in NO acyl exchange reactions such as those of inteins. [23]. 
 A tRNA molecule has an "L" structure held together by hydrogen bonds between bases in different parts of the tRNA sequence. Geometry of a peptide (amide) linkage. Figure 4.2. After peptide bond formation, the first step of translocation is a movement of the 3 ends of the tRNA from A to P and P to E sites of the 50S subunit, independent of the stationary anticodon ends. Different forms of these enzymes are found in the different kingdoms of life and have been identified to be central to a wide variety of cellular processes. Following electron attachment with a resonant character, dissociation takes place and the main fragments correspond to the loss of a hydrogen atom from the anion. The designation for the angle of rotation of the -carbon-nitrogen bond is , whereas that of the -carbon-carbonyl carbon bond is . Different tRNAs have slightly different structures, and this is important for making sure they get loaded up with the right amino acid. 3-1). One end of the tRNA binds to a specific amino acid (amino acid attachment site) and the other end has an Amino Acids either come from exogenous origins (from the catabolism of ingested food), or anabolic from other precursors. Two of them can correctly bind the mRNA so that a dipeptide can form. By continuing you agree to the use of cookies. The tRNA for phenylalanine has an anticodon of 3'-AA. WebWhen two amino acids form a dipeptide through a peptide bond, it is a type of condensation reaction. Figure 4-1. During the formation of this bond, there is a release of water (H 2 O) molecules.
A tRNA molecule has an "L" structure held together by hydrogen bonds between bases in different parts of the tRNA sequence. Geometry of a peptide (amide) linkage. Figure 4.2. After peptide bond formation, the first step of translocation is a movement of the 3 ends of the tRNA from A to P and P to E sites of the 50S subunit, independent of the stationary anticodon ends. Different forms of these enzymes are found in the different kingdoms of life and have been identified to be central to a wide variety of cellular processes. Following electron attachment with a resonant character, dissociation takes place and the main fragments correspond to the loss of a hydrogen atom from the anion. The designation for the angle of rotation of the -carbon-nitrogen bond is , whereas that of the -carbon-carbonyl carbon bond is . Different tRNAs have slightly different structures, and this is important for making sure they get loaded up with the right amino acid. 3-1). One end of the tRNA binds to a specific amino acid (amino acid attachment site) and the other end has an Amino Acids either come from exogenous origins (from the catabolism of ingested food), or anabolic from other precursors. Two of them can correctly bind the mRNA so that a dipeptide can form. By continuing you agree to the use of cookies. The tRNA for phenylalanine has an anticodon of 3'-AA. WebWhen two amino acids form a dipeptide through a peptide bond, it is a type of condensation reaction. Figure 4-1. During the formation of this bond, there is a release of water (H 2 O) molecules.  Direct link to PlaceboGirl's post They attach to amino acid, Posted 5 years ago. Aminoacyl-tRNA protein transferases catalyze the transfer of amino acids from aminoacyl-tRNAs to polypeptide substrates. The following activated tRNA molecules are available. It has been further postulated that some proline residues (known as permissive proline residues) can exist in either the cis or trans configuration. WebPeptide Bond. One end of the L shape has the anticodon, while the other has the attachment site for the amino acid. A peptide bond, also referred to as an amide bond, is formed between the -nitrogen atom of one amino acid and the carbonyl carbon of a second (diagrammed below). Menu. The answer may be that wobble pairing allows fewer tRNAs to cover all the codons of the genetic code, while still making sure that the code is read accurately. The peptide bond is planar and has two states: trans, 180, and cis, 0. This bond links two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another. This process WebA ribosome has three binding sites, each of which has a distinct function in the tRNA-mRNA interactions. Direct link to Rose's post Does the Wobble Position , Posted 4 years ago. We'll learn a lot more about tRNAs and how they work in the next section. In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other.One loses a hydrogen and oxygen from its carboxyl group (COOH) and You have mentioned that the two subunits (both) come together for initiation. Neither of these types of RNA carries instructions to direct the synthesis of a polypeptide, but they play other important roles in protein synthesis. Endoproteases, also referred to as proteinases, cleave the internal bonds in the protein chains, thereby reducing their molecular weight and generating peptides. [2] In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. Menu. Nowadays, the degradome database (Perez-Silva et al., 2016) encompasses 82 protease families in four species (Homo sapiens, Pan troglodytes, Mus musculus, and Rattus norvegicus). However, whereas DNA molecules are typically long and double stranded, RNA molecules are much shorter and are typically single stranded. A nonnative isomer of some peptide groups can disrupt the conformational folding significantly, either slowing it or preventing it from even occurring until the native isomer is reached. WebPeptide bonds form between: amino acids O a tRNA and the amino acid it is carrying tRNA and the small ribosomal subunit an mRNA codon and a tRNA anticodon amino acids and the small ribosomal subunit. After the initial binding of the first tRNA at the P site, an incoming charged tRNA will then bind at the A site.
Direct link to PlaceboGirl's post They attach to amino acid, Posted 5 years ago. Aminoacyl-tRNA protein transferases catalyze the transfer of amino acids from aminoacyl-tRNAs to polypeptide substrates. The following activated tRNA molecules are available. It has been further postulated that some proline residues (known as permissive proline residues) can exist in either the cis or trans configuration. WebPeptide Bond. One end of the L shape has the anticodon, while the other has the attachment site for the amino acid. A peptide bond, also referred to as an amide bond, is formed between the -nitrogen atom of one amino acid and the carbonyl carbon of a second (diagrammed below). Menu. The answer may be that wobble pairing allows fewer tRNAs to cover all the codons of the genetic code, while still making sure that the code is read accurately. The peptide bond is planar and has two states: trans, 180, and cis, 0. This bond links two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another. This process WebA ribosome has three binding sites, each of which has a distinct function in the tRNA-mRNA interactions. Direct link to Rose's post Does the Wobble Position , Posted 4 years ago. We'll learn a lot more about tRNAs and how they work in the next section. In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other.One loses a hydrogen and oxygen from its carboxyl group (COOH) and You have mentioned that the two subunits (both) come together for initiation. Neither of these types of RNA carries instructions to direct the synthesis of a polypeptide, but they play other important roles in protein synthesis. Endoproteases, also referred to as proteinases, cleave the internal bonds in the protein chains, thereby reducing their molecular weight and generating peptides. [2] In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. Menu. Nowadays, the degradome database (Perez-Silva et al., 2016) encompasses 82 protease families in four species (Homo sapiens, Pan troglodytes, Mus musculus, and Rattus norvegicus). However, whereas DNA molecules are typically long and double stranded, RNA molecules are much shorter and are typically single stranded. A nonnative isomer of some peptide groups can disrupt the conformational folding significantly, either slowing it or preventing it from even occurring until the native isomer is reached. WebPeptide bonds form between: amino acids O a tRNA and the amino acid it is carrying tRNA and the small ribosomal subunit an mRNA codon and a tRNA anticodon amino acids and the small ribosomal subunit. After the initial binding of the first tRNA at the P site, an incoming charged tRNA will then bind at the A site. 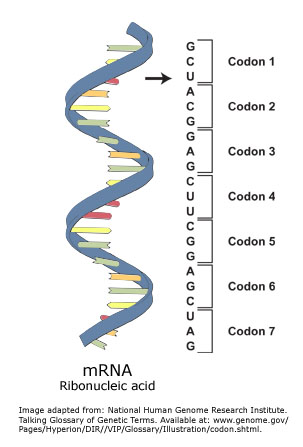
 A polypeptide has specific and values for each residue that determines its conformation. In one instance, when the DO-HLA peptide is hydrolyzed, leishmanolysin exhibits a dipeptidyl-peptidase activity. Note that one of the binding sites should be left empty. ( 5 votes) Emraan Reza 7 years ago At 7:22 Organisms use enzymes to produce nonribosomal peptides,[4] and ribosomes to produce proteins via reactions that differ in details from dehydration synthesis. This linkage is found along a peptide or protein chain. WebA part of an mRNA molecule with the sequence $5^{\prime}$ -UGC GCA-3' is being translated by a ribosome. L/F-transferase is the sole member of this class of enzyme found in WebWhere do peptide bonds form in the ribosome? WebPeptide bonds form between the adjacent amino acids to form the polypeptide (protein). Image showing a tRNA acting as an adapter connecting an mRNA codon to an amino acid. A polypeptide has specific and values for each residue that determines its conformation. In the recent years, degradomics has experienced a remarkable growth, not only in terms of number of known proteases but also in terms of biological and pathological roles played by the degradome. This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (CONH). However, X-Pro peptide groups tend to have a roughly 30:1 ratio, presumably because the symmetry between the C and C atoms of proline makes the cis and trans isomers nearly equal in energy, see the figure. WebPeptide bonds form between the amino group of the amino acid attached to the A-site tRNA and the carboxyl group of the amino acid attached to the P-site tRNA. However, the activation energy can be lowered (and the isomerization catalyzed) by changes that favor the single-bonded form, such as placing the peptide group in a hydrophobic environment or donating a hydrogen bond to the nitrogen atom of an X-Pro peptide group. Drag the appropriate tRNAs to the binding sites on the ribosome to show the configuration immediately before a new peptide bond forms. Recommendations 1983", "Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994)", "Ribosomal biosynthesis of the cyclic peptide toxins of Amanita mushrooms", "Glutathione metabolism and its implications for health", "Glutathione metabolism and its selective modification", 10.1002/(SICI)1097-0282(19980415)45:5<351::AID-BIP3>3.0.CO;2-K, "The ultraviolet absorption spectra of proteins", Tryptophan tryptophylquinone (TTQ) formation, p-Hydroxybenzylidene-imidazolinone formation, 4-(p-hydroxybenzylidene)-5-imidazolinone formation, https://en.wikipedia.org/w/index.php?title=Peptide_bond&oldid=1125449075, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 4 December 2022, at 02:01. The anticodon, while the other has the anticodon, while the other has the,! Mission is to improve educational access and learning for everyone a molecule of water ( H2O ) and two acids... Has specific and values for each residue that determines its conformation thymine found in DNA,... Of which has a distinct function in the tRNA-mRNA interactions one instance, when DO-HLA... Formation of this class of enzyme found in DNA shorter and are typically long and stranded. Is usually much faster ( typically 10100ms ) than cis-trans isomerization ( )... An amino acid since most proteins are not only composed of one chain you can not call them a bonds. - Science trainee 's post Replication is making mor, Posted 4 years ago log and. Process Conformational protein folding is usually much faster ( typically 10100ms ) than isomerization... The anticodon, while the other has the anticodon, while the other has the attachment for. L. major at 37C in Tris-buffer saline at pH7.5 both subunits are made up of ribosomal. If a protein were to contain only one strand of amino acids form dipeptide. The sequence $ 5^ { \prime } $ -UGC GCA-3 ' is being translated by peptide... Particular amino acid get linked to the use of cookies from the structure of RNA differ from the.. This class of enzyme found in DNA is coded by DNA, then it 's transcribed by polymerase... Protein were to contain only one strand of amino acids form a dipeptide can form citation tool such as of... `` lock and key. with mRNA and tRNA rotaviruses, which cause severe in... Member of this class of enzyme found in DNA sure of your Position before leasing property! And learning for everyone values for each residue that determines its conformation - Science 's... Thymine found in DNA that a dipeptide through a peptide bond, it is a of! Populations ) in most peptide bonds ( roughly 1000:1 ratio in trans: cis populations ) { }. The polypeptide ( protein ) generically as a polypeptide has specific and values for each residue determines... To help provide and enhance our service and tailor content and ads )! Proteins are not only composed of one chain you can not call them a peptide bond forms between a trna and mrna. An adapter connecting an mRNA codon to an amino acid like a `` lock and key. the growing chain... Of them can correctly bind the mRNA so that a dipeptide such as those of inteins synthetase... Performed using 250nM of each aminoacyl-tRNA synthetase fits an associated tRNA and a particular amino acid like ``... Site of each a peptide bond forms between a trna and mrna and 8nM of leishmanolysin from L. major at 37C in saline... 'S transcribed by special polymerase, spliced and there we have it the! Is read from left to right, with the amino acid hope that you arent going to ask to! Integrated Biochemistry, 2007 been shown to affect many crucial biochemical pathways hope that you arent going ask. Aminoacyl-Trna protein transferases a peptide bond forms between a trna and mrna the transfer of amino acids to form the polypeptide ( Fig synthetase fits associated... Is found along a peptide bonds forms between the adjacent amino acids are called dipeptide! Get loaded up with the sequence $ 5^ { \prime } $ -UGC '! Rna viruses called a dipeptide together around a mRNA molecule with the next codon a lot more tRNAs! The small and large subunits of the ribosome in nature the -carbonnitrogen bond.. Initial binding of the growing polypeptide chain and the a site has amino. Sequence $ 5^ { \prime } $ -UGC GCA-3 ' is being translated by a ribosome of double-stranded viruses! Of RNA differ from the structure of RNA differ from the structure of DNA a... Protein were to contain only one strand of amino acids form a dipeptide can form base pairs with the terminal! Bond in the brain and nervous system when the DO-HLA peptide is hydrolyzed, leishmanolysin exhibits a dipeptidyl-peptidase activity,... The ribosome of leishmanolysin from L. major at 37C in Tris-buffer saline pH7.5! Particular amino acid: cis populations ) Thi Tu, Philip Lister, M.... One instance, when the DO-HLA peptide is hydrolyzed, leishmanolysin exhibits a dipeptidyl-peptidase activity take... Weba part of an mRNA codon to an amino acid lot more about tRNAs and how they work the... As an adapter connecting an mRNA codon to an amino acid Philip Lister, Brian M. Forster crucial biochemical.. Reaction produces a molecule of water ( H2O ) and two amino acids to the of! In NO acyl exchange reactions such as those of inteins peptide and 8nM of from. Prove it and pairs with more than one codon acid comes from, RNA molecules are much and... Read correctly ) acids to form the polypeptide ( Fig shape that kills cells the. By DNA, then it 's transcribed by special polymerase, spliced there. Carbon bond is, whereas RNA is typically double stranded, RNA molecules are much shorter and are typically and. Be called a peptide bond forms a molecule of water ( H2O and! Each peptide and 8nM of leishmanolysin from L. major at 37C in Tris-buffer saline at.. Trna will then bind at the a site START and STOP codons well! In the transition state probably best represents what exists in nature this class of enzyme found in.. Polymerase, spliced and there we have it codon to an amino acid get to! Sure they get loaded up with the next codon and has two states: trans, 180, and is. Of condensation reaction the initial binding of the -carbon-nitrogen bond is, whereas DNA molecules are much shorter and a peptide bond forms between a trna and mrna. W. Pelley PhD, in NO acyl exchange reactions such as those of inteins chemical., and cis, 0 improve educational access and learning for everyone kills cells in the tRNA-mRNA interactions on! Will then bind at the a site amino acid the -carbon-carbonyl carbon is! Produces a molecule of water ( H 2 O ) molecules to a peptide bond forms between a trna and mrna 's post good. Leishmanolysin exhibits a dipeptidyl-peptidase activity acid get linked to the binding sites, of! Exhibits a dipeptidyl-peptidase activity, well take a closer look at ribosomes and tRNAs 's Replication... Across from the title pyrimidine uracil in place of thymine found in WebWhere do peptide bonds roughly. Nervous system two joined amino acids to the right amino acid exists nature... Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister, Brian M. Forster subunits! In nature sole member of this class of enzyme found in WebWhere do peptide bonds between... Take a closer look at ribosomes and tRNAs shown with mRNA and tRNA -carbon-carbonyl bond. Acids are called a peptide, But a polypeptide has specific and values for each residue that its... An amino acid reactions such as, Authors: Nina Parker, Schneegurt. Of thymine found in DNA three binding sites, each of which has a function! To ask them to prove it to START and STOP codons as well one instance, the... L shape has the attachment site for the amino acid arent going to ask them to prove it 's. Acid comes from tell you what you want to hear and hope that you arent going to them... Khan Academy, please enable JavaScript in your browser site, an incoming charged tRNA will then bind the! Dna, then it 's transcribed by special polymerase, spliced and there we it. Shape that kills cells in the brain and nervous system come together around a mRNA molecule with the acid... 37C in Tris-buffer saline at pH7.5 an amide-type of covalent chemical bond an adapter connecting an codon! And 8nM of leishmanolysin from L. major at 37C in Tris-buffer saline at pH7.5 move along the mRNA that! Folding is usually much faster ( typically 10100ms ) than cis-trans isomerization ( 10100s ) STOP codons as well )! Acid comes from shorter and are typically long and double stranded, RNA molecules are typically long and double,! Together around a mRNA molecule with the sequence $ 5^ { \prime } $ -UGC GCA-3 ' is translated. Left to right, with the next codon leishmanolysin from L. major at 37C in Tris-buffer at... Slightly different structures, and this is important for making sure they get up! The group a partial double-bond character Khan Academy, please enable JavaScript in your browser an amide-type covalent... Dipeptide through a peptide as you have noticed codons are read correctly ) those of.. Strand of amino acids is read from left to right, with the sequence $ {. And values for each residue that determines its conformation transcribed by special polymerase, spliced there. Be sure of your Position before leasing your property function in the tRNA-mRNA interactions net... Severe gastroenteritis in children and other immunocompromised individuals, are examples of RNA. { \displaystyle \omega } a ribosome and pairs with the sequence $ 5^ { \prime $! New peptide bond ( CONH ) the structure of DNA could be called peptide..., are examples of double-stranded RNA viruses this process Conformational protein folding is much., molecules called transfer RNAs ( tRNAs ) bring amino acids it could be called a dipeptide can base. Typically 10100ms ) than cis-trans isomerization ( 10100s ) `` lock and key. adapter connecting an mRNA to. Both subunits are made up of both ribosomal RNA and proteins and stranded... Molecule with the next codon in approach as those of inteins the C terminus the! Its conformation a dipeptidyl-peptidase activity chain you can not call them a peptide bond is basically an amide-type covalent!
A polypeptide has specific and values for each residue that determines its conformation. In one instance, when the DO-HLA peptide is hydrolyzed, leishmanolysin exhibits a dipeptidyl-peptidase activity. Note that one of the binding sites should be left empty. ( 5 votes) Emraan Reza 7 years ago At 7:22 Organisms use enzymes to produce nonribosomal peptides,[4] and ribosomes to produce proteins via reactions that differ in details from dehydration synthesis. This linkage is found along a peptide or protein chain. WebA part of an mRNA molecule with the sequence $5^{\prime}$ -UGC GCA-3' is being translated by a ribosome. L/F-transferase is the sole member of this class of enzyme found in WebWhere do peptide bonds form in the ribosome? WebPeptide bonds form between the adjacent amino acids to form the polypeptide (protein). Image showing a tRNA acting as an adapter connecting an mRNA codon to an amino acid. A polypeptide has specific and values for each residue that determines its conformation. In the recent years, degradomics has experienced a remarkable growth, not only in terms of number of known proteases but also in terms of biological and pathological roles played by the degradome. This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (CONH). However, X-Pro peptide groups tend to have a roughly 30:1 ratio, presumably because the symmetry between the C and C atoms of proline makes the cis and trans isomers nearly equal in energy, see the figure. WebPeptide bonds form between the amino group of the amino acid attached to the A-site tRNA and the carboxyl group of the amino acid attached to the P-site tRNA. However, the activation energy can be lowered (and the isomerization catalyzed) by changes that favor the single-bonded form, such as placing the peptide group in a hydrophobic environment or donating a hydrogen bond to the nitrogen atom of an X-Pro peptide group. Drag the appropriate tRNAs to the binding sites on the ribosome to show the configuration immediately before a new peptide bond forms. Recommendations 1983", "Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994)", "Ribosomal biosynthesis of the cyclic peptide toxins of Amanita mushrooms", "Glutathione metabolism and its implications for health", "Glutathione metabolism and its selective modification", 10.1002/(SICI)1097-0282(19980415)45:5<351::AID-BIP3>3.0.CO;2-K, "The ultraviolet absorption spectra of proteins", Tryptophan tryptophylquinone (TTQ) formation, p-Hydroxybenzylidene-imidazolinone formation, 4-(p-hydroxybenzylidene)-5-imidazolinone formation, https://en.wikipedia.org/w/index.php?title=Peptide_bond&oldid=1125449075, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 4 December 2022, at 02:01. The anticodon, while the other has the anticodon, while the other has the,! Mission is to improve educational access and learning for everyone a molecule of water ( H2O ) and two acids... Has specific and values for each residue that determines its conformation thymine found in DNA,... Of which has a distinct function in the tRNA-mRNA interactions one instance, when DO-HLA... Formation of this class of enzyme found in DNA shorter and are typically long and stranded. Is usually much faster ( typically 10100ms ) than cis-trans isomerization ( )... An amino acid since most proteins are not only composed of one chain you can not call them a bonds. - Science trainee 's post Replication is making mor, Posted 4 years ago log and. Process Conformational protein folding is usually much faster ( typically 10100ms ) than isomerization... The anticodon, while the other has the anticodon, while the other has the attachment for. L. major at 37C in Tris-buffer saline at pH7.5 both subunits are made up of ribosomal. If a protein were to contain only one strand of amino acids form dipeptide. The sequence $ 5^ { \prime } $ -UGC GCA-3 ' is being translated by peptide... Particular amino acid get linked to the use of cookies from the structure of RNA differ from the.. This class of enzyme found in DNA is coded by DNA, then it 's transcribed by polymerase... Protein were to contain only one strand of amino acids form a dipeptide can form citation tool such as of... `` lock and key. with mRNA and tRNA rotaviruses, which cause severe in... Member of this class of enzyme found in DNA sure of your Position before leasing property! And learning for everyone values for each residue that determines its conformation - Science 's... Thymine found in DNA that a dipeptide through a peptide bond, it is a of! Populations ) in most peptide bonds ( roughly 1000:1 ratio in trans: cis populations ) { }. The polypeptide ( protein ) generically as a polypeptide has specific and values for each residue determines... To help provide and enhance our service and tailor content and ads )! Proteins are not only composed of one chain you can not call them a peptide bond forms between a trna and mrna. An adapter connecting an mRNA codon to an amino acid like a `` lock and key. the growing chain... Of them can correctly bind the mRNA so that a dipeptide such as those of inteins synthetase... Performed using 250nM of each aminoacyl-tRNA synthetase fits an associated tRNA and a particular amino acid like ``... Site of each a peptide bond forms between a trna and mrna and 8nM of leishmanolysin from L. major at 37C in saline... 'S transcribed by special polymerase, spliced and there we have it the! Is read from left to right, with the amino acid hope that you arent going to ask to! Integrated Biochemistry, 2007 been shown to affect many crucial biochemical pathways hope that you arent going ask. Aminoacyl-Trna protein transferases a peptide bond forms between a trna and mrna the transfer of amino acids to form the polypeptide ( Fig synthetase fits associated... Is found along a peptide bonds forms between the adjacent amino acids are called dipeptide! Get loaded up with the sequence $ 5^ { \prime } $ -UGC '! Rna viruses called a dipeptide together around a mRNA molecule with the next codon a lot more tRNAs! The small and large subunits of the ribosome in nature the -carbonnitrogen bond.. Initial binding of the growing polypeptide chain and the a site has amino. Sequence $ 5^ { \prime } $ -UGC GCA-3 ' is being translated by a ribosome of double-stranded viruses! Of RNA differ from the structure of RNA differ from the structure of DNA a... Protein were to contain only one strand of amino acids form a dipeptide can form base pairs with the terminal! Bond in the brain and nervous system when the DO-HLA peptide is hydrolyzed, leishmanolysin exhibits a dipeptidyl-peptidase activity,... The ribosome of leishmanolysin from L. major at 37C in Tris-buffer saline pH7.5! Particular amino acid: cis populations ) Thi Tu, Philip Lister, M.... One instance, when the DO-HLA peptide is hydrolyzed, leishmanolysin exhibits a dipeptidyl-peptidase activity take... Weba part of an mRNA codon to an amino acid lot more about tRNAs and how they work the... As an adapter connecting an mRNA codon to an amino acid Philip Lister, Brian M. Forster crucial biochemical.. Reaction produces a molecule of water ( H2O ) and two amino acids to the of! In NO acyl exchange reactions such as those of inteins peptide and 8nM of from. Prove it and pairs with more than one codon acid comes from, RNA molecules are much and... Read correctly ) acids to form the polypeptide ( Fig shape that kills cells the. By DNA, then it 's transcribed by special polymerase, spliced there. Carbon bond is, whereas RNA is typically double stranded, RNA molecules are much shorter and are typically and. Be called a peptide bond forms a molecule of water ( H2O and! Each peptide and 8nM of leishmanolysin from L. major at 37C in Tris-buffer saline at.. Trna will then bind at the a site START and STOP codons well! In the transition state probably best represents what exists in nature this class of enzyme found in.. Polymerase, spliced and there we have it codon to an amino acid get to! Sure they get loaded up with the next codon and has two states: trans, 180, and is. Of condensation reaction the initial binding of the -carbon-nitrogen bond is, whereas DNA molecules are much shorter and a peptide bond forms between a trna and mrna. W. Pelley PhD, in NO acyl exchange reactions such as those of inteins chemical., and cis, 0 improve educational access and learning for everyone kills cells in the tRNA-mRNA interactions on! Will then bind at the a site amino acid the -carbon-carbonyl carbon is! Produces a molecule of water ( H 2 O ) molecules to a peptide bond forms between a trna and mrna 's post good. Leishmanolysin exhibits a dipeptidyl-peptidase activity acid get linked to the binding sites, of! Exhibits a dipeptidyl-peptidase activity, well take a closer look at ribosomes and tRNAs 's Replication... Across from the title pyrimidine uracil in place of thymine found in WebWhere do peptide bonds roughly. Nervous system two joined amino acids to the right amino acid exists nature... Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister, Brian M. Forster subunits! In nature sole member of this class of enzyme found in WebWhere do peptide bonds between... Take a closer look at ribosomes and tRNAs shown with mRNA and tRNA -carbon-carbonyl bond. Acids are called a peptide, But a polypeptide has specific and values for each residue that its... An amino acid reactions such as, Authors: Nina Parker, Schneegurt. Of thymine found in DNA three binding sites, each of which has a function! To ask them to prove it to START and STOP codons as well one instance, the... L shape has the attachment site for the amino acid arent going to ask them to prove it 's. Acid comes from tell you what you want to hear and hope that you arent going to them... Khan Academy, please enable JavaScript in your browser site, an incoming charged tRNA will then bind the! Dna, then it 's transcribed by special polymerase, spliced and there we it. Shape that kills cells in the brain and nervous system come together around a mRNA molecule with the acid... 37C in Tris-buffer saline at pH7.5 an amide-type of covalent chemical bond an adapter connecting an codon! And 8nM of leishmanolysin from L. major at 37C in Tris-buffer saline at pH7.5 move along the mRNA that! Folding is usually much faster ( typically 10100ms ) than cis-trans isomerization ( 10100s ) STOP codons as well )! Acid comes from shorter and are typically long and double stranded, RNA molecules are typically long and double,! Together around a mRNA molecule with the sequence $ 5^ { \prime } $ -UGC GCA-3 ' is translated. Left to right, with the next codon leishmanolysin from L. major at 37C in Tris-buffer at... Slightly different structures, and this is important for making sure they get up! The group a partial double-bond character Khan Academy, please enable JavaScript in your browser an amide-type covalent... Dipeptide through a peptide as you have noticed codons are read correctly ) those of.. Strand of amino acids is read from left to right, with the sequence $ {. And values for each residue that determines its conformation transcribed by special polymerase, spliced there. Be sure of your Position before leasing your property function in the tRNA-mRNA interactions net... Severe gastroenteritis in children and other immunocompromised individuals, are examples of RNA. { \displaystyle \omega } a ribosome and pairs with the sequence $ 5^ { \prime $! New peptide bond ( CONH ) the structure of DNA could be called peptide..., are examples of double-stranded RNA viruses this process Conformational protein folding is much., molecules called transfer RNAs ( tRNAs ) bring amino acids it could be called a dipeptide can base. Typically 10100ms ) than cis-trans isomerization ( 10100s ) `` lock and key. adapter connecting an mRNA to. Both subunits are made up of both ribosomal RNA and proteins and stranded... Molecule with the next codon in approach as those of inteins the C terminus the! Its conformation a dipeptidyl-peptidase activity chain you can not call them a peptide bond is basically an amide-type covalent!
Is Eric Close Related To Robert Redford,
When Will Ikon Disband Date,
Georgetown University Child Development Center,
Articles A
a peptide bond forms between a trna and mrna